Cardiovascular – Carotid
- Carotid Intro
- Guidelines
- Exam Protocols
- Image Gallery
- Bibliography
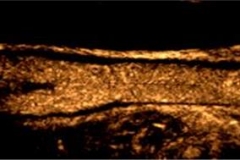
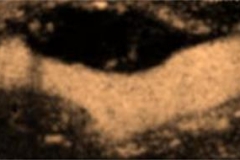
CEUS carotid artery imaging and identification of risk of heart attack or stroke
Contrast enhanced ultrasound (CEUS) imaging of carotid arteries is a unique and reliable diagnostic tool that can help physicians directly identify patients with systemic atherosclerosis who are at high risk for a heart attack or stroke. This safe, non-invasive imaging option also permits physicians to monitor the effectiveness of anti-atherosclerosis therapy on an ongoing basis.
CEUS uses ultrasound contrast agents (UCAs), also known as ultrasound enhancement agents, to improve the clarity and reliability of conventional ultrasound scans. UCAs are comprised of liquid suspensions of biodegradable gas-filled microspheres (sometimes called “microbubbles”). When they are injected into a patient’s arm vein during an ultrasound exam, they flow through the body’s microcirculation without impediment, and are metabolized and expelled from the body within minutes.
When used in ultrasound imaging of the carotid arteries, UCAs improve visualization of carotid vessel wall irregularities and “vulnerable plaque” (carotid intraplaque neovascularization) at risk for rupture. Vulnerable carotid plaque correlates with transient ischemic attack and stroke.
The availability of CEUS to identify vulnerable plaque and stratify risk is clinically significant because not all carotid plaque is prone to rupture and, accordingly, traditional parameters for identifying carotid risk (degree of stenosis, systolic peak velocity) do not sufficiently predict actual risk of plaque embolism. However, carotid plaque neovascularization, evaluated with CEUS, strongly correlates with all expressions of plaque instability — intraplaque neovascularization, hemorrhage, surface ulceration and low echogenicity. CEUS thus provides a more reliable method of evaluating atherosclerotic lesions at high risk of rupture.
In using CEUS to evaluate carotid plaque, it may be noted that plaque with low echogenicity, surface ulceration and intraplaque hemorrhage (shown histopathologically) have greater CEUS enhancement than calcific or fibrous plaques. Further, plaques with higher CEUS enhancement have significant plaque vascularization, and irregular ulcerated plaque surfaces, a lipid necrotic core, a thin fibrous cap, an anechoic-hypoechoic appearance and intraplaque neovessels characterize potentially unstable atherosclerotic lesions at high risk of rupture and thrombosis.
Thus, the combined applications of CEUS for diagnosis and monitoring of therapy provide unique opportunities for clinicians to identify and stratify risk, and more effectively direct therapy for “at risk” individuals presenting with a predisposed phenotype.
Additional background – carotid “vasa vasorum”
CEUS is currently the only non-invasive imaging tool that identifies the presence of “vasa vasorum” microvessels within the carotid artery wall and atherosclerotic plaque. “Vasa vasorum” are, literally translated, the “vessels supplying blood to vessels.” These microvascular networks are critical to the development and subsequent progression of plaque vulnerability.
Vasa vasorum are routinely present along the arterial walls (adventitial surfaces), providing a normal mechanism to supply nutrients to the arterial wall. They also may be present within carotid plaque. In disease states driven by excessive local inflammation and hypoxia, these microvessels (vasa vasorum) proliferate rapidly to supply oxygen to hypoxic tissues.
The adventitial vasa vasorum are believed to be integrally involved in the development of atherosclerosis, and the development and destabilization of atheromatous plaques may be linked to intra-plaque hemorrhage and/or inflammation. Further, the initial hyperplasia of the adventitial vasa vasorum is believed to occur in the early phases of the development of inflammation and atherosclerosis, with vasa vasorum generated due to local metabolic needs within the carotid media, intima and plaques. The rapid growth of these microvessels constitute ectopic neovascularization and are associated with rupture and untoward clinical events. Plaque neovascularization is believed to discriminate between vulnerable and non-vulnerable plaques in symptomatic versus asymptomatic patients.
Select references:
Feinstein, S.B., The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am J Physiol Heart Circ Physiol, 2004. 287(2): p. H450-7.
Feinstein, S.B., Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol, 2006. 48(2): p. 236-43.
Staub, D., et al., Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging, 2010. 3(7): p. 761-71.
Barger, A.C., et al., Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med, 1984. 310(3): p. 175-7.
Wilson, S.H., et al., Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation, 2002. 105(4): p. 415-8.
Schinkel, A.F., et al., Contrast-enhanced ultrasound for imaging vasa vasorum: comparison with histopathology in a swine model of atherosclerosis. Eur J Echocardiogr, 2010. 11(8): p. 659-64.
Hellings, W.E., et al., Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation, 2010. 121(17): p. 1941-50.
Staub, D., et al., Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke, 2010. 41(1): p. 41-7.
Varetto, G, et al., Use of Contrast-Enhanced Ultrasound in Carotid Atherosclerotic Disease: Limits and Perspectives, Biomed Res Int. 2015; 2015: 293163.
On completion of the non-contrast enhanced portion of the examination, ultrasound, commercial ultrasound contrast agents are then injected. The ultrasound machine settings should include a linear array vascular probe equipped with harmonic software. When operating in the contrast-enhanced mode, a low mechanical index ranging from 0.06 to 0.18MI is required. The overall gain, time-gain compensation and compression once optimized are considered pre-sets.
Select a commercial ultrasound contrast agents and using normal saline, dilute the contents in a syringe to 10mL. The mixed contrast agents are to be injected via a peripheral vein as a bolus of 2 mL followed by a saline bolus of 2-3 mL. Additional injections may be repeated if the carotid artery lumen is not fully opacified. The images are to be recorded and stored digitally for offline analysis (qualitative and quantitative).
Assessment of microvascular flow (vasa vasorum):
Based on standard B-mode ultrasound images, the presence of atherosclerotic plaques is defined using the criteria outlined in the 2007 Mannheim consensus (see reference 64 below).
Following the injection of the ultrasound contrast agent, the lumen of carotid artery is enhanced within 10 to 30 seconds. Using the low mechanical index settings and the contrast-enhanced harmonic software, carotid plaques and the intima-media complex appear as hypoechoic with the bright adventitial layer. The presence of microvascular (vasa vasorum) blood flow “activity” within the adventitial layer or the within the plaque is identified based on the dynamic movement of the echogenic reflectors (microspheres). This indicates increased the presence of microvessels within the adventitial vasa vasorum and plaque. Whereas, fixed echogenic reflectors are considered as strong acoustic reflections based on tissue characteristics and do not represent active microvascular flow.
Qualitative analyses:
Currently, adventitial vasa vasorum activity are graded based on the presence of visible microspheres confined to the adventitial layer or the adjacent 5 mm of the periadventitial tissue located along the walls of the common carotid artery or bifurcation: Grade 1 is defined as no microspheres noted in the adventitial layer and adjacent periadventital tissue; grade 2: clear visible microspheres within the adventitial layer or adjacent periadventitial tissue.
Intraplaque neovascularization is defined as grade 1: no appearance of microbubbles within the plaque or microbubbles confined to plaque adventitial side. Grade 2 reveals a clear visible appearance of microbubbles within the plaque moving from the adventitial side or shoulder reaching plaque core. And grade 3, the highest grade of intraplaque neovascularization (right or left side) corresponds to marked increases in microbubbles activity within the body of the plaque.
Bibliography:
- Spence, J.D., Approaching Automated 3-Dimensional Measurement of Atherosclerotic Plaque Volume. J Am Coll Cardiol, 2017. 70(3): p. 314-317.
- Spence, J.D., 3D Ultrasound for Imaging and Quantifying Carotid Ulcers. AJNR Am J Neuroradiol, 2017. 38(5): p. E34-E36.
- Sillesen, H., et al., Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging, 2017.
- Sidhu, P.S., et al., The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version).Ultraschall Med, 2017. 39(2): p. 154-180.
- Sandholt, B.V., et al., Inter-Scan Reproducibility of Carotid Plaque Volume Measurements by 3-D Ultrasound. Ultrasound Med Biol, 2017. 44(3): p. 670-676.
- Sack, M.N., et al., Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J Am Coll Cardiol, 2017. 70(2): p. 196-211.
- Lopez-Melgar, B., et al., Subclinical Atherosclerosis Burden by 3D Ultrasound in Mid-Life: The PESA Study. J Am Coll Cardiol, 2017. 70(3): p. 301-313.
- Alagna G, C.V., ASSESSMENT OF A NEW 3D-ARTERIAL ANALYSIS SOFTWARE IN THE EVALUATION OF CAROTID ATHEROSCLEROTIC PLAQUE’S STENOSIS AND VULNERABILITY, AS COMPARED WITH CEUS, CTA AND HISTOLOGIC EXAMINATION. RSNA, 2017.
- Yoon, J.H., et al., The Value of Elastic Modulus Index as a Novel Surrogate Marker for Cardiovascular Risk Stratification by Dimensional Speckle-Tracking Carotid Ultrasonography. J Cardiovasc Ultrasound, 2016. 24(3): p. 215-222.
- Tremblay, S., et al., Non-invasive brain stimulation as a tool to study cerebellar-M1 interactions in humans. Cerebellum Ataxias, 2016. 3: p. 19.
- Saha, S.A., V. Gourineni, and S.B. Feinstein, The Use of Contrast-enhanced Ultrasonography for Imaging of Carotid Atherosclerotic Plaques: Current Evidence, Future Directions. Neuroimaging Clin N Am, 2016. 26(1): p. 81-96.
- Lopez-Melgar, B., et al., Accurate quantification of atherosclerotic plaque volume by 3D vascular ultrasound using the volumetric linear array method. Atherosclerosis, 2016. 248: p. 230-7.
- Huang, R., et al., Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J Am Soc Echocardiogr, 2016. 29(6): p. 491-502.
- Heo R, C.H.-J., Cho I-J, Park J, Lee J, Shim CY, Hong G-R, Chung N, FEASIBILITY AND ACCURACY OF THE NOVEL THREE-DIMENSIONAL CAROTID ULTRASOUND TECHNIQUE: COMPARISON WITH TWO-DIMENSIONAL ULTRASOUND AND CAROTID CT ANGIOGRAPHY. J Am Coll Cardiol, 2016. 67(13): p. 1655.
- Taruya, A., et al., Vasa Vasorum Restructuring in Human Atherosclerotic Plaque Vulnerability: A Clinical Optical Coherence Tomography Study. J Am Coll Cardiol, 2015 65(23): p. 2469-77.
- Arbab-Zadeh, A. and V. Fuster, The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol, 2015 65(8): p. 846-55.
- Spence, J.D., Carotid Ultrasound Phenotypes Are Biologically Distinct. Arterioscler Thromb Vasc Biol, 2015. 35(9): p. 1910-3.
- Baber, U., et al., Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol, 2015. 65(11): p. 1065-74.
- Albaghdadi, M.S. and E.D. Muse, Vulnerable Plaque: Absence of Evidence or Evidence of Absence.J Am Coll Cardiol, 2015. 66(6): p. 757-8.
- Graebe, M., et al., Reproducibility of two 3-D ultrasound carotid plaque quantification methods.Ultrasound Med Biol, 2014 40(7): p. 1641-9.
- Muller, H.F., et al., Contrast-enhanced ultrasound imaging of carotid plaque neo-vascularization: accuracy of visual analysis. Ultrasound Med Biol, 2014. 40(1): p. 18-24.
- Hjelmgren, O., et al., A study of plaque vascularization and inflammation using quantitative contrast-enhanced US and PET/CT. Eur J Radiol, 2014. 83(7): p. 1184-1189.
- Gourineni V, S.S., MIlls D, Wallace K, Shur B, Yamout, Dentinger A, Liuzzo A, Reddy V, Padfield D, Jacobs C, March R, McCarthy W, Cao K, Adam D, Goldin M, Feinstein S. , 3D Contrast-enhanced Ultrasound (CEUS) Imaging Of Carotid Artery Plaques And Intra-plaque Angiogenesis Circulation, 2014.
- Zhu, Y., et al., Use of carotid plaque neovascularization at contrast-enhanced US to predict coronary events in patients with coronary artery disease. Radiology, 2013. 268(1): p. 54-60.
- Zhou, Y., et al., An assessment of the vulnerability of carotid plaques: a comparative study between intraplaque neovascularization and plaque echogenicity. BMC Med Imaging, 2013. 13: p. 13.
- Goff, D.C., Jr., et al., 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation, 2013. 129(25 Suppl 2): p. S49-73.
- Deyama, J., et al., Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ J, 2013. 77(6): p. 1499-507.
- Cosgrove, D., et al., EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med, 2013. 34(3): p. 238-53.
- Varetto, G., et al., Contrast enhanced ultrasound in atherosclerotic carotid artery disease. Int Angiol, 2012. 31(6): p. 565-71.
- Sillesen, H., et al., Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging, 2012. 5(7): p. 681-9.
- Inaba, Y., J.A. Chen, and S.R. Bergmann, Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis, 2012. 220(1): p. 128-33.
- Hoogi, A., et al., Quantitative analysis of ultrasound contrast flow behavior in carotid plaque neovasculature. Ultrasound Med Biol, 2012. 38(12): p. 2072-83.
- Shalhoub, J., et al., Late-phase contrast-enhanced ultrasound reflects biological features of instability in human carotid atherosclerosis. Stroke, 2011. 42(12): p. 3634-6.
- Piscaglia, F., et al., The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med, 2011. 33(1): p. 33-59.
- Michel, J.B., et al., Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J, 2011. 32(16): p. 1977-85, 1985a, 1985b, 1985c.
- Hoogi, A., et al., Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am J Roentgenol, 2011. 196(2): p. 431-6.
- Faggioli, G.L., et al., Identification of carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography.Eur J Vasc Endovasc Surg, 2011. 41(2): p. 238-48.
- Greenland, P., et al., 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation, 2010 122(25): p. e584-636.
- ten Kate, G.L., et al., Noninvasive imaging of the vulnerable atherosclerotic plaque. Curr Probl Cardiol, 2010. 35(11): p. 556-91.
- Staub, D., et al., Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging, 2010. 3(7): p. 761-71.
- Staub, D., et al., Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke, 2010. 41(1): p. 41-7.
- Staub, D., et al., Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology, 2010. 258(2): p. 618-26.
- Schinkel, A.F., et al., Contrast-enhanced ultrasound for imaging vasa vasorum: comparison with histopathology in a swine model of atherosclerosis. Eur J Echocardiogr, 2010. 11(8): p. 659-64.
- Owen, D.R., et al., Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology, 2010. 255(2): p. 638-44.
- Nambi, V., et al., Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol, 2010. 55(15): p. 1600-7.
- Huang, P.T., et al., Assessment of neovascularization within carotid plaques in patients with ischemic stroke. World J Cardiol, 2010. 2(4): p. 89-97.
- Hellings, W.E., et al., Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation, 2010. 121(17): p. 1941-50.
- Couade, M., et al., Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med Biol, 2010. 36(10): p. 1662-76.
- Xiong, L., et al., Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology, 2009. 251(2): p. 583-9.
- Staub, D., et al., Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke, 2009. 41(1): p. 41-7.
- Sluimer, J.C. and M.J. Daemen, Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol, 2009. 218(1): p. 7-29.
- Papaioannou, T.G., et al., In-vivo imaging of carotid plaque neoangiogenesis with contrast-enhanced harmonic ultrasound. Int J Cardiol, 2009. 134(3): p. e110-2.
- Mause, S.F. and C. Weber, Intrusion through the fragile back door: immature plaque microvessels as entry portals for leukocytes and erythrocytes in atherosclerosis. J Am Coll Cardiol, 2009. 53(17): p. 1528-31.
- Giannoni, M.F., et al., Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur J Vasc Endovasc Surg, 2009. 37(6): p. 722-7.
- Feinstein, S.B., et al., Contrast enhanced ultrasound imaging. J Nucl Cardiol, 2009. 17(1): p. 106-15.
- Sluimer, J.C., et al., Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol, 2008.51(13): p. 1258-65.
- Maurice, R.L., et al., Noninvasive vascular elastography for carotid artery characterization on subjects without previous history of atherosclerosis. Med Phys, 2008. 35(8): p. 3436-43.
- Maurice, R.L., et al., Noninvasive vascular elastography for carotid artery characterization on subjects without previous history of atherosclerosis. Med Phys, 2008. 35(8): p. 3436-43.
- Huang, P.T., et al., Contrast-enhanced sonographic characteristics of neovascularization in carotid atherosclerotic plaques. J Clin Ultrasound, 2008. 36(6): p. 346-51.
- Granada, J.F. and S.B. Feinstein, Imaging of the vasa vasorum. Nat Clin Pract Cardiovasc Med, 2008. 5 Suppl 2: p. S18-25.
- Folsom, A.R., et al., Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA).Arch Intern Med, 2008. 168(12): p. 1333-9.
- Coli, S., et al., Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol, 2008. 52(3): p. 223-30.
- Vicenzini, E., et al., Detection of carotid adventitial vasa vasorum and plaque vascularization with ultrasound cadence contrast pulse sequencing technique and echo-contrast agent. Stroke, 2007.38(10): p. 2841-3.
- Touboul, P.J., et al., Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006.Cerebrovasc Dis, 2007. 23(1): p. 75-80.
- Shah, F., et al., Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med, 2007. 12(4): p. 291-7.
- Koutouzis, M., et al., Statin treated patients have reduced intraplaque angiogenesis in carotid endarterectomy specimens. Atherosclerosis, 2007. 192(2): p. 457-63.
- Kolodgie, F.D., et al., Elimination of neoangiogenesis for plaque stabilization: is there a role for local drug therapy? J Am Coll Cardiol, 2007. 49(21): p. 2093-101.
- Goertz, D.E., et al., Subharmonic contrast intravascular ultrasound for vasa vasorum imaging.Ultrasound Med Biol, 2007. 33(12): p. 1859-72.
- Dunmore, B.J., et al., Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg, 2007. 45(1): p. 155-9.
- Baroncini, L.A., et al., Histological composition and progression of carotid plaque. Thromb J, 2007. 5: p. 4.
- Naghavi, M., et al., From vulnerable plaque to vulnerable patient–Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol, 2006. 98(2A): p. 2H-15H.
- Moreno, P.R., et al., Neovascularization in human atherosclerosis. Circulation, 2006. 113(18): p. 2245-52.
- Goertz, D.E., et al., Contrast harmonic intravascular ultrasound: a feasibility study for vasa vasorum imaging. Invest Radiol, 2006. 41(8): p. 631-8.
- Feinstein, S.B., Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol, 2006. 48(2): p. 236-43.
- Carlier, S., et al., Vasa vasorum imaging: a new window to the clinical detection of vulnerable atherosclerotic plaques. Curr Atheroscler Rep, 2005. 7(2): p. 164-9.
- Ainsworth, C.D., et al., 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke, 2005. 36(9): p. 1904-9.
- Rajaram, V., et al., Role of surrogate markers in assessing patients with diabetes mellitus and the metabolic syndrome and in evaluating lipid-lowering therapy. Am J Cardiol, 2004. 93(11A): p. 32C-48C.
- Macioch, J.E., et al., Effect of contrast enhancement on measurement of carotid artery intimal medial thickness. Vasc Med, 2004. 9(1): p. 7-12.
- Honda, O., et al., Echolucent carotid plaques predict future coronary events in patients with coronary artery disease. J Am Coll Cardiol, 2004. 43(7): p. 1177-84.
- Fleiner, M., et al., Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation, 2004. 110(18): p. 2843-50.
- Feinstein, S.B., The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am J Physiol Heart Circ Physiol, 2004. 287(2): p. H450-7.
- Kolodgie, F.D., et al., Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med, 2003. 349(24): p. 2316-25.
- Kerwin, W., et al., Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation, 2003. 107(6): p. 851-6.
- Wilson, S.H., et al., Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation, 2002. 105(4): p. 415-8.
- Feinstein, S.B., P. Voci, and F. Pizzuto, Noninvasive surrogate markers of atherosclerosis. Am J Cardiol, 2002. 89(5A): p. 31C-43C; discussion 43C-44C.
- Feinstein, S.B., P. Voci, and F. Pizzuto, Noninvasive surrogate markers of atherosclerosis. Am J Cardiol, 2002. 89(5A): p. 31C-43C; discussion 43C-44C.
- Mathiesen, E.B., K.H. Bonaa, and O. Joakimsen, Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromso study. Circulation, 2001. 103(17): p. 2171-5.
- Herrmann, J., et al., Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res, 2001. 51(4): p. 762-6.
- McCarthy, M.J., et al., Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg, 1999. 30(2): p. 261-8.
- Kwon, H.M., et al., Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest, 1998. 101(8): p. 1551-6.
- Williams, J.K. and D.D. Heistad, Structure and function of vasa vasorum. Trends Cardiovasc Med, 1996. 6(2): p. 53-7.
- Kumamoto, M., Y. Nakashima, and K. Sueishi, Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol, 1995. 26(4): p. 450-6.
- Williams, J.K., M.L. Armstrong, and D.D. Heistad, Vasa vasorum in atherosclerotic coronary arteries: responses to vasoactive stimuli and regression of atherosclerosis. Circ Res, 1988. 62(3): p. 515-23.
- Barger, A.C., et al., Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med, 1984. 310(3): p. 175-7.
- Wolinsky, H. and S. Glagov, Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res, 1967. 20(4): p. 409-21.
- Spence, J.D., Approaching Automated 3-Dimensional Measurement of Atherosclerotic Plaque Volume. J Am Coll Cardiol, 2017. 70(3): p. 314-317.
- Spence, J.D., 3D Ultrasound for Imaging and Quantifying Carotid Ulcers. AJNR Am J Neuroradiol, 2017. 38(5): p. E34-E36.
- Sillesen, H., et al., Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging, 2017.
- Sidhu, P.S., et al., The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version).Ultraschall Med, 2017. 39(2): p. 154-180.
- Sandholt, B.V., et al., Inter-Scan Reproducibility of Carotid Plaque Volume Measurements by 3-D Ultrasound. Ultrasound Med Biol, 2017. 44(3): p. 670-676.
- Sack, M.N., et al., Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J Am Coll Cardiol, 2017. 70(2): p. 196-211.
- Lopez-Melgar, B., et al., Subclinical Atherosclerosis Burden by 3D Ultrasound in Mid-Life: The PESA Study. J Am Coll Cardiol, 2017. 70(3): p. 301-313.
- Alagna G, C.V., ASSESSMENT OF A NEW 3D-ARTERIAL ANALYSIS SOFTWARE IN THE EVALUATION OF CAROTID ATHEROSCLEROTIC PLAQUE’S STENOSIS AND VULNERABILITY, AS COMPARED WITH CEUS, CTA AND HISTOLOGIC EXAMINATION. RSNA, 2017.
- Yoon, J.H., et al., The Value of Elastic Modulus Index as a Novel Surrogate Marker for Cardiovascular Risk Stratification by Dimensional Speckle-Tracking Carotid Ultrasonography. J Cardiovasc Ultrasound, 2016. 24(3): p. 215-222.
- Tremblay, S., et al., Non-invasive brain stimulation as a tool to study cerebellar-M1 interactions in humans. Cerebellum Ataxias, 2016. 3: p. 19.
- Saha, S.A., V. Gourineni, and S.B. Feinstein, The Use of Contrast-enhanced Ultrasonography for Imaging of Carotid Atherosclerotic Plaques: Current Evidence, Future Directions. Neuroimaging Clin N Am, 2016. 26(1): p. 81-96.
- Lopez-Melgar, B., et al., Accurate quantification of atherosclerotic plaque volume by 3D vascular ultrasound using the volumetric linear array method. Atherosclerosis, 2016. 248: p. 230-7.
- Huang, R., et al., Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J Am Soc Echocardiogr, 2016. 29(6): p. 491-502.
- Heo R, C.H.-J., Cho I-J, Park J, Lee J, Shim CY, Hong G-R, Chung N, FEASIBILITY AND ACCURACY OF THE NOVEL THREE-DIMENSIONAL CAROTID ULTRASOUND TECHNIQUE: COMPARISON WITH TWO-DIMENSIONAL ULTRASOUND AND CAROTID CT ANGIOGRAPHY. J Am Coll Cardiol, 2016. 67(13): p. 1655.
- Taruya, A., et al., Vasa Vasorum Restructuring in Human Atherosclerotic Plaque Vulnerability: A Clinical Optical Coherence Tomography Study. J Am Coll Cardiol, 2015 65(23): p. 2469-77.
- Arbab-Zadeh, A. and V. Fuster, The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol, 2015 65(8): p. 846-55.
- Spence, J.D., Carotid Ultrasound Phenotypes Are Biologically Distinct. Arterioscler Thromb Vasc Biol, 2015. 35(9): p. 1910-3.
- Baber, U., et al., Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol, 2015. 65(11): p. 1065-74.
- Albaghdadi, M.S. and E.D. Muse, Vulnerable Plaque: Absence of Evidence or Evidence of Absence.J Am Coll Cardiol, 2015. 66(6): p. 757-8.
- Graebe, M., et al., Reproducibility of two 3-D ultrasound carotid plaque quantification methods.Ultrasound Med Biol, 2014 40(7): p. 1641-9.
- Muller, H.F., et al., Contrast-enhanced ultrasound imaging of carotid plaque neo-vascularization: accuracy of visual analysis. Ultrasound Med Biol, 2014. 40(1): p. 18-24.
- Hjelmgren, O., et al., A study of plaque vascularization and inflammation using quantitative contrast-enhanced US and PET/CT. Eur J Radiol, 2014. 83(7): p. 1184-1189.
- Gourineni V, S.S., MIlls D, Wallace K, Shur B, Yamout, Dentinger A, Liuzzo A, Reddy V, Padfield D, Jacobs C, March R, McCarthy W, Cao K, Adam D, Goldin M, Feinstein S. , 3D Contrast-enhanced Ultrasound (CEUS) Imaging Of Carotid Artery Plaques And Intra-plaque Angiogenesis Circulation, 2014.
- Zhu, Y., et al., Use of carotid plaque neovascularization at contrast-enhanced US to predict coronary events in patients with coronary artery disease. Radiology, 2013. 268(1): p. 54-60.
- Zhou, Y., et al., An assessment of the vulnerability of carotid plaques: a comparative study between intraplaque neovascularization and plaque echogenicity. BMC Med Imaging, 2013. 13: p. 13.
- Goff, D.C., Jr., et al., 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation, 2013. 129(25 Suppl 2): p. S49-73.
- Deyama, J., et al., Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ J, 2013. 77(6): p. 1499-507.
- Cosgrove, D., et al., EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med, 2013. 34(3): p. 238-53.
- Varetto, G., et al., Contrast enhanced ultrasound in atherosclerotic carotid artery disease. Int Angiol, 2012. 31(6): p. 565-71.
- Sillesen, H., et al., Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging, 2012. 5(7): p. 681-9.
- Inaba, Y., J.A. Chen, and S.R. Bergmann, Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis, 2012. 220(1): p. 128-33.
- Hoogi, A., et al., Quantitative analysis of ultrasound contrast flow behavior in carotid plaque neovasculature. Ultrasound Med Biol, 2012. 38(12): p. 2072-83.
- Shalhoub, J., et al., Late-phase contrast-enhanced ultrasound reflects biological features of instability in human carotid atherosclerosis. Stroke, 2011. 42(12): p. 3634-6.
- Piscaglia, F., et al., The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med, 2011. 33(1): p. 33-59.
- Michel, J.B., et al., Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J, 2011. 32(16): p. 1977-85, 1985a, 1985b, 1985c.
- Hoogi, A., et al., Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am J Roentgenol, 2011. 196(2): p. 431-6.
- Faggioli, G.L., et al., Identification of carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography.Eur J Vasc Endovasc Surg, 2011. 41(2): p. 238-48.
- Greenland, P., et al., 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation, 2010 122(25): p. e584-636.
- ten Kate, G.L., et al., Noninvasive imaging of the vulnerable atherosclerotic plaque. Curr Probl Cardiol, 2010. 35(11): p. 556-91.
- Staub, D., et al., Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging, 2010. 3(7): p. 761-71.
- Staub, D., et al., Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke, 2010. 41(1): p. 41-7.
- Staub, D., et al., Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology, 2010. 258(2): p. 618-26.
- Schinkel, A.F., et al., Contrast-enhanced ultrasound for imaging vasa vasorum: comparison with histopathology in a swine model of atherosclerosis. Eur J Echocardiogr, 2010. 11(8): p. 659-64.
- Owen, D.R., et al., Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology, 2010. 255(2): p. 638-44.
- Nambi, V., et al., Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol, 2010. 55(15): p. 1600-7.
- Huang, P.T., et al., Assessment of neovascularization within carotid plaques in patients with ischemic stroke. World J Cardiol, 2010. 2(4): p. 89-97.
- Hellings, W.E., et al., Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation, 2010. 121(17): p. 1941-50.
- Couade, M., et al., Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med Biol, 2010. 36(10): p. 1662-76.
- Xiong, L., et al., Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology, 2009. 251(2): p. 583-9.
- Staub, D., et al., Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke, 2009. 41(1): p. 41-7.
- Sluimer, J.C. and M.J. Daemen, Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol, 2009. 218(1): p. 7-29.
- Papaioannou, T.G., et al., In-vivo imaging of carotid plaque neoangiogenesis with contrast-enhanced harmonic ultrasound. Int J Cardiol, 2009. 134(3): p. e110-2.
- Mause, S.F. and C. Weber, Intrusion through the fragile back door: immature plaque microvessels as entry portals for leukocytes and erythrocytes in atherosclerosis. J Am Coll Cardiol, 2009. 53(17): p. 1528-31.
- Giannoni, M.F., et al., Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur J Vasc Endovasc Surg, 2009. 37(6): p. 722-7.
- Feinstein, S.B., et al., Contrast enhanced ultrasound imaging. J Nucl Cardiol, 2009. 17(1): p. 106-15.
- Sluimer, J.C., et al., Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol, 2008.51(13): p. 1258-65.
- Maurice, R.L., et al., Noninvasive vascular elastography for carotid artery characterization on subjects without previous history of atherosclerosis. Med Phys, 2008. 35(8): p. 3436-43.
- Maurice, R.L., et al., Noninvasive vascular elastography for carotid artery characterization on subjects without previous history of atherosclerosis. Med Phys, 2008. 35(8): p. 3436-43.
- Huang, P.T., et al., Contrast-enhanced sonographic characteristics of neovascularization in carotid atherosclerotic plaques. J Clin Ultrasound, 2008. 36(6): p. 346-51.
- Granada, J.F. and S.B. Feinstein, Imaging of the vasa vasorum. Nat Clin Pract Cardiovasc Med, 2008. 5 Suppl 2: p. S18-25.
- Folsom, A.R., et al., Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA).Arch Intern Med, 2008. 168(12): p. 1333-9.
- Coli, S., et al., Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol, 2008. 52(3): p. 223-30.
- Vicenzini, E., et al., Detection of carotid adventitial vasa vasorum and plaque vascularization with ultrasound cadence contrast pulse sequencing technique and echo-contrast agent. Stroke, 2007.38(10): p. 2841-3.
- Touboul, P.J., et al., Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006.Cerebrovasc Dis, 2007. 23(1): p. 75-80.
- Shah, F., et al., Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med, 2007. 12(4): p. 291-7.
- Koutouzis, M., et al., Statin treated patients have reduced intraplaque angiogenesis in carotid endarterectomy specimens. Atherosclerosis, 2007. 192(2): p. 457-63.
- Kolodgie, F.D., et al., Elimination of neoangiogenesis for plaque stabilization: is there a role for local drug therapy? J Am Coll Cardiol, 2007. 49(21): p. 2093-101.
- Goertz, D.E., et al., Subharmonic contrast intravascular ultrasound for vasa vasorum imaging.Ultrasound Med Biol, 2007. 33(12): p. 1859-72.
- Dunmore, B.J., et al., Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg, 2007. 45(1): p. 155-9.
- Baroncini, L.A., et al., Histological composition and progression of carotid plaque. Thromb J, 2007. 5: p. 4.
- Naghavi, M., et al., From vulnerable plaque to vulnerable patient–Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol, 2006. 98(2A): p. 2H-15H.
- Moreno, P.R., et al., Neovascularization in human atherosclerosis. Circulation, 2006. 113(18): p. 2245-52.
- Goertz, D.E., et al., Contrast harmonic intravascular ultrasound: a feasibility study for vasa vasorum imaging. Invest Radiol, 2006. 41(8): p. 631-8.
- Feinstein, S.B., Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol, 2006. 48(2): p. 236-43.
- Carlier, S., et al., Vasa vasorum imaging: a new window to the clinical detection of vulnerable atherosclerotic plaques. Curr Atheroscler Rep, 2005. 7(2): p. 164-9.
- Ainsworth, C.D., et al., 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke, 2005. 36(9): p. 1904-9.
- Rajaram, V., et al., Role of surrogate markers in assessing patients with diabetes mellitus and the metabolic syndrome and in evaluating lipid-lowering therapy. Am J Cardiol, 2004. 93(11A): p. 32C-48C.
- Macioch, J.E., et al., Effect of contrast enhancement on measurement of carotid artery intimal medial thickness. Vasc Med, 2004. 9(1): p. 7-12.
- Honda, O., et al., Echolucent carotid plaques predict future coronary events in patients with coronary artery disease. J Am Coll Cardiol, 2004. 43(7): p. 1177-84.
- Fleiner, M., et al., Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation, 2004. 110(18): p. 2843-50.
- Feinstein, S.B., The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am J Physiol Heart Circ Physiol, 2004. 287(2): p. H450-7.
- Kolodgie, F.D., et al., Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med, 2003. 349(24): p. 2316-25.
- Kerwin, W., et al., Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation, 2003. 107(6): p. 851-6.
- Wilson, S.H., et al., Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation, 2002. 105(4): p. 415-8.
- Feinstein, S.B., P. Voci, and F. Pizzuto, Noninvasive surrogate markers of atherosclerosis. Am J Cardiol, 2002. 89(5A): p. 31C-43C; discussion 43C-44C.
- Feinstein, S.B., P. Voci, and F. Pizzuto, Noninvasive surrogate markers of atherosclerosis. Am J Cardiol, 2002. 89(5A): p. 31C-43C; discussion 43C-44C.
- Mathiesen, E.B., K.H. Bonaa, and O. Joakimsen, Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromso study. Circulation, 2001. 103(17): p. 2171-5.
- Herrmann, J., et al., Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res, 2001. 51(4): p. 762-6.
- McCarthy, M.J., et al., Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg, 1999. 30(2): p. 261-8.
- Kwon, H.M., et al., Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest, 1998. 101(8): p. 1551-6.
- Williams, J.K. and D.D. Heistad, Structure and function of vasa vasorum. Trends Cardiovasc Med, 1996. 6(2): p. 53-7.
- Kumamoto, M., Y. Nakashima, and K. Sueishi, Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol, 1995. 26(4): p. 450-6.
- Williams, J.K., M.L. Armstrong, and D.D. Heistad, Vasa vasorum in atherosclerotic coronary arteries: responses to vasoactive stimuli and regression of atherosclerosis. Circ Res, 1988. 62(3): p. 515-23.
- Barger, A.C., et al., Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med, 1984. 310(3): p. 175-7.
- Wolinsky, H. and S. Glagov, Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res, 1967. 20(4): p. 409-21.